About Mangalam Drugs & Organics Ltd
Mangalam Drugs & Organics Limited commenced its manufacturing of Active Pharmaceutical Ingredients (APIs) and Intermediates at VAPI – Gujarat in 1977. It has a multi-product manufacturing facility on two locations, and an in-house Research & Development laboratory recognized by the Department of Scientific & Industrial Research, Delhi Government of India (DSIR).
Over the last five decades, Mangalam has acquired a worldwide reputation as a single stop destination for frontline Anti-Malaria APIs. We also have a diversified product range having synergies in operations and economies of scale. Mangalam is amongst the top companies in Asia in all the products it makes; and is also the largest manufacturer of some of its products in the world.
Over the last five decades, Mangalam has acquired a worldwide reputation as a single stop destination for frontline Anti-Malaria APIs. We also have a diversified product range having synergies in operations and economies of scale. Mangalam is amongst the top companies in Asia in all the products it makes; and is also the largest manufacturer of some of its products in the world.
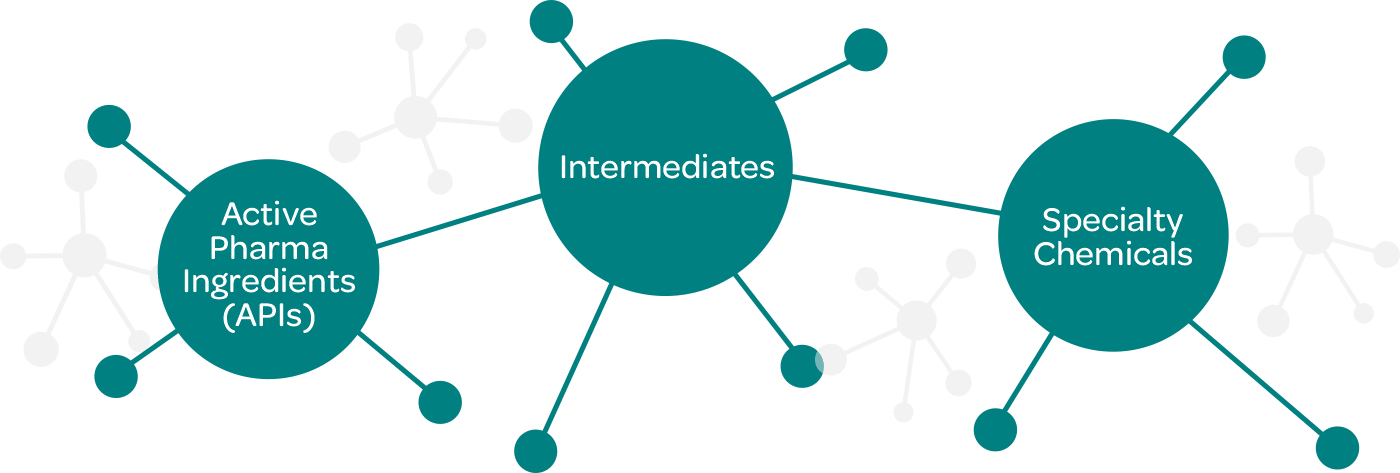
We are presently focused in the manufacturing of the following


Along with the WHO-GENEVA GMP, MFDS Korea
certification, EDQM, ANVISA approval for its API
manufacturing plants, the company is also an agreement
with the prestigious Clinton Health Access Initiative
(CHAI) under its Fight Malaria Program for suplly of Anti-
Malaria APIs worldwide.

Vision & Mission
To be a persistently customer engaged API manufacturing company in the healthcare business and thereby achieving growth in the industry.


Quality Policy
We are committed to satisfy our customers’ needs & expectations. We understand & believe we can meet commitments to customers by
Board of Directors

Accreditations








